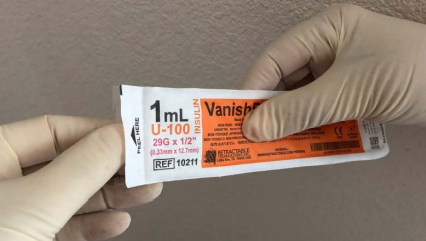
Vanishpoint Online
Training Content
Welcome to the Vanishpoint online training portal
This landing page will act as your training course for the Vanishpoint safety needles and syringe range, we have developed this online training portal to support with the reconstitution/dilution of the COVID-19 vaccine. Once you have reviewed all of the training content below then please fill in the attendance form at the bottom of the page, this will act as a registration of you reviewing and understanding the content provided and will be fed back to a member of your team.
Table of Contents
Section 1) Video: Introduction to the Vanishpoint Product
Section 2) Opening the Vanishpoint Syringe
Section 3) Process for dilution/reconstitution
Section 4) Video: Demonstration of the reconstitution/dilution process using a Vanishpoint syringe
Section 5) Removal of the air bubble
Section 6) Video: Demonstration of the air bubble being removed
Section 7) How to activate the retraction mechanism
Section 8) Explanation/Information regarding the Vanishpoint syringe barrel lubrication
Section 1) Video: Introduction to the Vanishpoint Product
Section 2) Opening the Vanishpoint Syringe
View the selection of images below, that illustrates how to open the packaging of the Vanishpoint safety syringe, it is important to remember that you should not force the syringe through the packaging.
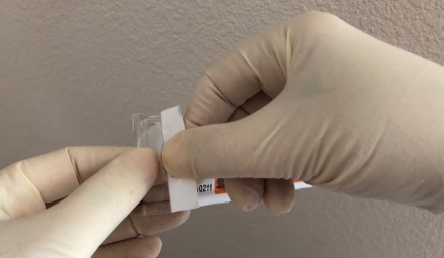
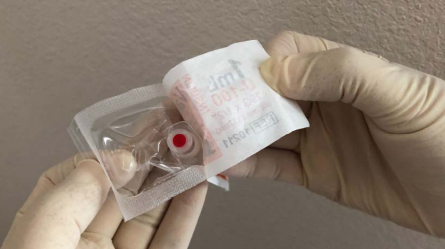
Section 3) Process for dilution/reconstitution
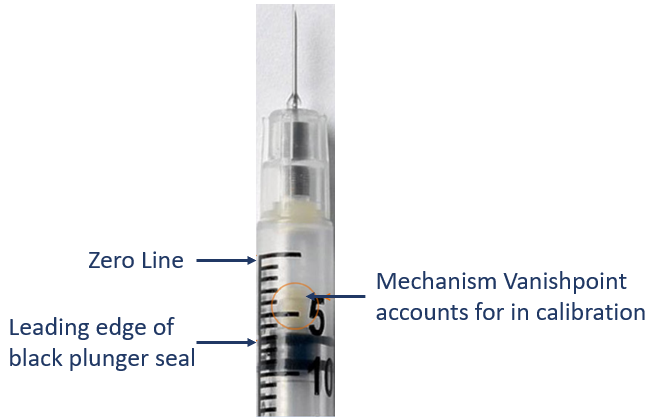
- The top line denotes zero
- Draw up the dose as you normally would
- Measure using the leading edge of the black plunger seal
- VanishPoint syringes have been calibrated to account for the retraction mechanism on top of the plunger seal
- Rotate the plunger handle as you slowly push up to the correct dose
- Once the correct dose of diluent has been measured add air
- Inject diluent slowly into the vial of medication to be reconstituted
- The air cushion will allow all of the diluent to be expelled from the syringe without activating the retraction mechanism. STOP pushing the plunger at the resistance point to avoid activating the retraction mechanism.
- Withdraw air to equalise vial
- Remove the needle from the vial and activate retraction mechanism
Section 4) Video: Demonstration of the reconstitution/dilution process using a Vanishpoint syringe
Section 5) Removal of the Air Bubble
- When preparing medication dose, remove bubbles as much as possible
- Due to the unique safety design, there may be a small air bubble,
trapped below the spring area but above the zero line, which can be
resistant to removal - This small, trapped bubble will not be injected into the patient and will not
affect the dose accuracy - VanishPoint syringes have verified dose accuracy in multiple dosages,
including small doses such as 1 unit (0.01mL) and2 units (0.02 mL),
with and without a small air bubble present
Tips to assist with bubble removal from a Vanishpoint safety syringe, as seen in the video demonstration below:
- Draw up the dose as you normally would, tap syringe barrel to dislodge and consolidate air bubbles at the top (needle end) of the syringe
- Once bubbles have risen to the needle end of the syringe, draw the plunger handle back to create a small air pocket, as shown in the video below
- Keeping the syringe level, rotate the plunger handle as you slowly push up to the correct dosage
- NOTE: At times, a small air bubble, below the spring area but above the zero line, may be resistant to removal. This small, trapped bubble will not affect the dose accuracy (see above information).
Section 6) Video: Demonstration of the air bubble being removed
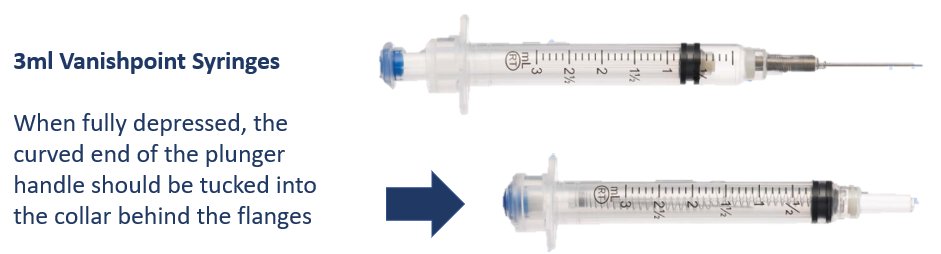
Section 7) How to activate the retraction mechanism
Fully depress the plunger handle to deliver the full dose and activate the retraction
Section 8) Explanation/Information regarding the Vanishpoint syringe barrel lubricant
G&N have had frequently asked questions regarding the lubricant inside the Vanishpoint syringe barrel. The following bullet points detail the key information on the Vanishpoint syringe barrel lubricant.
- The interior barrel surfaces of all VanishPoint syringes are lubricated with a medical-grade lubricant made from medical grade silicone fluid (360 Medical Fluid – 12,500 cSt).
- This lubricant is necessary to improve the ease of plunger handle movement, and does not pose a danger to the patient or the healthcare worker.
- VanishPoint barrel lubrication is inspected by Process Control and Quality Assurance during and after the manufacturing process
to ensure compliance with the ISO 7886-1:1993 specification, which states that the quantity of lubricant shall not exceed 0.25mg/cm2
of the interior surface area of the syringe barrel. - Due to the unique retraction mechanism, which is activated when the plunger handle is fully depressed, at the point of manufacture the VanishPoint plunger handle cannot be fully exercised (or pushed to the end of the barrel) after application of the lubricant.
- . The VanishPoint plunger handle is inserted to approximately 75% of the barrel length to prevent premature activation of the needle retraction mechanism.
- . The result is a small but sometimes visible settlement line of lubricant inside the syringe barrel, which is dispersed upon plunger movement.
510000/1